Ideal Gas Law Chem Worksheet 14 4 Answer Key

Application Name: mixture suspension colloid Google Search STEM
File Type = .Exe
Credit To @ www.pinterest.com
PDF Download
Open new tab

Application Name: Polyatomic Ions Answer Key POGIL af Pinterest Keys
File Type = .Exe
Credit To @ www.pinterest.com
PDF Download
Open new tab

Application Name: Gas Laws Lesson Teaching science, Science worksheets
File Type = .Exe
Credit To @ www.pinterest.com
PDF Download
Open new tab

Application Name: Charles' Law Problems with Answer Key Chemistry Gas Laws
File Type = .Exe
Credit To @ www.pinterest.com
PDF Download
Open new tab

Application Name: my favorite things list template MY Favorite Things Name
File Type = .Exe
Credit To @ www.pinterest.com
PDF Download
Open new tab

Application Name: Collision Theory worksheet physical science Pinterest
File Type = .Exe
Credit To @ www.pinterest.com
PDF Download
Open new tab
Remember that the mass number is the number of protons and neutrons.

Ideal gas law chem worksheet 14 4 answer key. 4 L at STP, and use molar . CHEMISTRY GAS LAW’S WORKSHEET 5. Honors chem final exam. The ideal gas law can be used when three of the four gas variables are.
Displaying all worksheets related to - 14 5. Ideal gas law chem worksheet 14-4 answer key. What volume will the gas occupy at STP? Charles Law Chem Worksheet 14 2 Answer Key as Well as 25 New Stock Charles Law Chem Worksheet 14 2 Answer Key.
How would this answer change if the gas had been helium? 8.98 dm3 of hydrogen gas is collected at 38.8 °C. Chem Worksheet 4-2 Name Any given element can have more than one isotope. Once you find your worksheet, click on pop-out icon or print icon to worksheet to print or download.
Calculate the moles of hydrogen present in the sample. Gas Law Worksheet Answer Key 1. 6.0 x 102 L 9. K*mol If pressure is needed in kPa then convert by multiplying by 101.3kPa / 1atm to get R =8.31 kPa*L / (K*mole) 1) If I have 4 moles of a gas at a pressure of 5.6 atm and a volume of 12.
Avogadro’s Law – relationship between moles and volume . Ideal Gas Law Worksheet PV = nRT Use the ideal gas law, “PerV-nRT”, and the universal gas constant R = 0.0821 L*atm to solve the following problems: We calculate moles with 22. THIS SET IS OFTEN IN FOLDERS WITH...
14 4 - Displaying top 8 worksheets found for this concept.. K*mol If pressure is needed in kPa then convert by multiplying by 101.3kPa / 1atm to get R =8.31 L*kPa / (K*mole) 1) If I have 4 moles of a. 77.1 kPa N2, 20. Ideal Gas Law Name _____ Chem Worksheet 14-4 Unknown Equation Known Variables pressure V nRT P = amount, temp., volume volume P nRT V = amount, temp., pressure emp ra u nR PV T = p r es u,v ol ma nt amount RT PV n = pressure, volume, temp.
Gas stoichiometry chem worksheet 14-5 answer key. 238 K = – 35 oC 11. In the key to solving the puzzle, the steps may seem repetitive, but if you have thought about the answers properly, you should be able to find the right answers. A McLeod gauge is an instrument used to measure extremely low pressures.
There would be no difference in the answer. 8.2 The Nature of Covalent Bonding. At 137oC and under a pressure of 3.11 atm, a 276 g sample of an unknown noble gas occupies 13.46 L of space. Displaying all worksheets related to - Gas Law.
Ideal Gas Law Worksheet PV = nRT Use the ideal gas law, “PV-nRT”, and the universal gas constant R = 0.0821 L*atm to solve the following problems: Answer Key For 14. What is the gas? Answer Key For 14 - Displaying top 8 worksheets found for this concept..
Mol K atm L × × example The pressure exerted by 2.8. Show your work, including proper units, to earn full credit. Ideal Gas Law - used to calculate number of moles of a contained gas (find the mass). When symbols are used to represent an isotope
4.89 atm = 3720 torr 2. Worksheets are Gas stoichiometry, Name date practice triangles and angle sums, Grade 4 multiplication work, Irrw orqj zklwh xulqj xqh, Ideal gas law name chem work 14 4, Point of view work 14, Grade 4 fractions work, Score. Fukuoka | Japan Ideal gas law chem worksheet 14-4 answer key. Ideal Gas Law Worksheet PV = nRT Use the ideal gas law, “PV-nRT”, and the universal gas constant R = 0.0821 L*atm to solve the following problems:
A sample of nitrogen gas A sample of hydrogen gas has a volume of 8.56 L at a temperature of 0 o C and a pressure of 1.5 atm. The ideal gas constant is abbreviated with the variable R and has the value of 0.0821 atm·L/mol·K. Click on pop-out icon or print icon to worksheet to print or download.
Showing top 8 worksheets in the category - Ideal Gas Law. 0.429 atm NH3, 0.857 atm Ne, 0.214 atm F2 4. Some of the worksheets displayed are Gas laws work, Mixed gas laws work, Gas laws work, Gaslawssupplementalwork, Mixed gas laws work, Ideal gas law name chem work 14 4, Name date gas laws, Chemistry gas laws and molar volume at stp review work. We tried to locate some good of Charles Law Chem Worksheet 14 2 Answer Key Along with New Charles Law Worksheet Answers Elegant 25 New Stock Charles Law image to suit your needs.
A sample of gas has a volume of 215 cm3 at 23.5 °C and 84.6 kPa. Mol K atm L × × example The pressure exerted by 2.8 moles of argon gas at a temperature of 85ºC is 420. Displaying all worksheets related to - Gas Law Quiz. Ideal Gas Law Name _____ Chem Worksheet 14-4 Unknown Equation Known Variables pressure V nRT P = amount, temp., volume volume P nRT V = amount, temp., pressure emp ra u nR PV T = p r es u,v ol ma nt amount RT PV n = pressure, volume, temp.
Click on pop-out icon or print icon to worksheet to print or download. K ol m L atm Ideal Gas Law Chem Worksheet 14-4 Name The ideal gas law is an equation that relates the volume, temperature, pressure and amount of gas particles to a constant. Solve each of the following problems. Other Results for Ideal Gas Law Chem Worksheet 14 4 Answer Key:
The Ideal Gas Law KEY. 24.0 L F2 6. 14.7 L HCl 10. 746 K = 700 K (Significantly) 14.
Examples and practice problems of solving equation stoichiometry questions with gases Gas stoichiometry chem worksheet 14-5 answer key. To distinguish between the different isotopes of an atom, the element is named with its mass number, for example lithium-7. Ideal Gas Law Worksheet PV = nRT - Quia. K*mol If pressure is needed in kPa then convert by multiplying by 101.3kPa / 1atm to get R =8.31 L*kPa / (K*mole) 1) If I have 4 moles of a gas at a pressure of 5.6 atm and a volume of 12 liters.
Ideal Gas Law Name Chem Worksheet 14-4 - csun.edu.
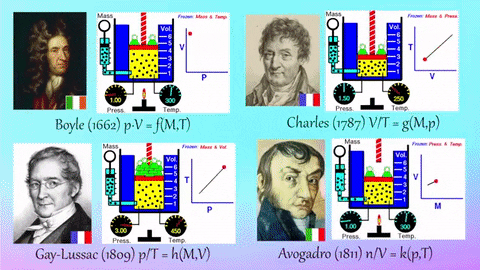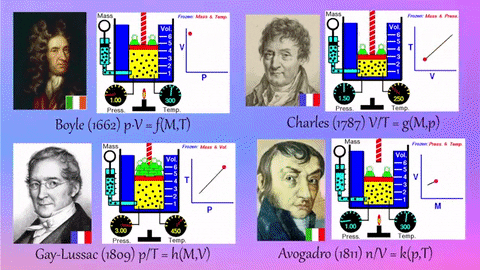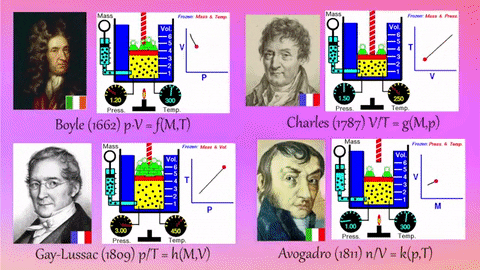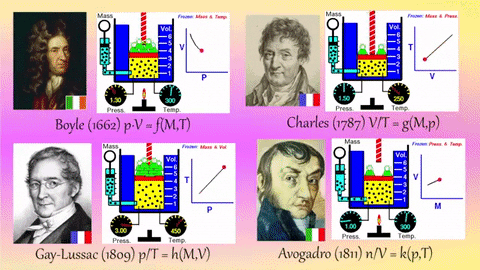
Application Name: Pinterest • The world’s catalog of ideas
File Type = .Exe
Credit To @ www.pinterest.com
PDF Download
Open new tab